Chemistry, 05.09.2019 18:30 ghwolf4p0m7x0
In solid nacl, the equilibrium separation between neighboring na+ and cl- ions is 0.283 nm. calculate the coulombic energy between na+ and at this distance. give your answer in each of j, ev, and kj/mol units.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1


Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
In solid nacl, the equilibrium separation between neighboring na+ and cl- ions is 0.283 nm. calculat...
Questions in other subjects:



History, 12.11.2020 02:20

Mathematics, 12.11.2020 02:20


English, 12.11.2020 02:20

Mathematics, 12.11.2020 02:20

Mathematics, 12.11.2020 02:20

Mathematics, 12.11.2020 02:20

History, 12.11.2020 02:20

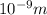 .
.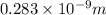
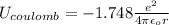
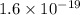 C
C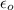 =
= 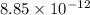
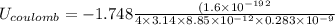
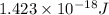
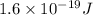
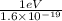
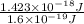
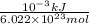
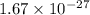 kJ/mol
kJ/mol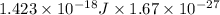 kJ/mol
kJ/mol