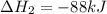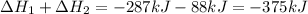Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2


Chemistry, 22.06.2019 19:30, liyahlanderson2232
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
Calculate δh⁰298 (in kj) for the process p(s) + 5/2 cl2(g) → pcl5(g) from the following information....
Questions in other subjects:

English, 24.01.2022 23:50





Physics, 24.01.2022 23:50




Mathematics, 24.01.2022 23:50

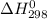 for the process is -375 kJ
for the process is -375 kJ .
.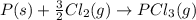 ......
......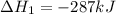
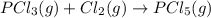 ......
......