Chemistry, 05.09.2019 18:30 itzdryoshi
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming of natural gas, in a two-step process. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 3h2(g) > 2nh3(g) in the second step, ammonia and oxygen react to form nitric acid and water: nh3(g) + 2o2(g) > hno3(g) + h2o(g) write the net chemical equation for the production of nitric acid from nitrogen, hydrogen and oxygen. be sure your equation is balanced.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, earcake2470
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1


Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prep...
Questions in other subjects:

Mathematics, 13.07.2019 18:00


Mathematics, 13.07.2019 18:00




Mathematics, 13.07.2019 18:00


History, 13.07.2019 18:00

Spanish, 13.07.2019 18:00

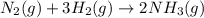 ......(1)
......(1)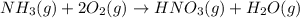 .....(2)
.....(2)