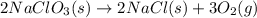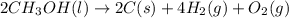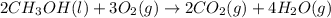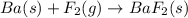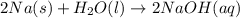Chemistry, 05.09.2019 17:20 skatingby8910
Classify each of the following reactions: i. decopositionii. combinationiii. combustion a. 2ch3oh(l)+3o2(g)→2co2(g)+4h2o(g) b. 2naclo3(s)→2nacl(s)+3o2(g) c. ba(s)+f2(g)→baf2(s) d. 2na(s)+h2o(l)→2naoh(aq) e. 2ch3oh(l)→2c(s)+4h2(g)+o2(g)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
Classify each of the following reactions: i. decopositionii. combinationiii. combustion a. 2ch3oh(l)...
Questions in other subjects:




Mathematics, 05.10.2020 18:01

Mathematics, 05.10.2020 18:01

Engineering, 05.10.2020 18:01


History, 05.10.2020 18:01

Spanish, 05.10.2020 18:01

Social Studies, 05.10.2020 18:01