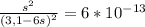Chemistry, 05.09.2019 17:10 wheeler2455
Nickel (ii) ions form a complex ion in the presence of ammonia with a formation constant (kf) of 2.0×10^8:
ni2+ + 6nh3 ⇌ [ni(nh3)6]2+
calculate the molar solubility of nis in 3.1 m nh3. g

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hannacarroll2539
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 03:00, HHHHHHHHHMMMMMMMMM
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3
You know the right answer?
Nickel (ii) ions form a complex ion in the presence of ammonia with a formation constant (kf) of 2.0...
Questions in other subjects:


Mathematics, 17.11.2020 01:00


Mathematics, 17.11.2020 01:00


Mathematics, 17.11.2020 01:00


Mathematics, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00

![\frac{[S^{2-}][Ni(NH3)6^{+2}]}{[NH3]^{6}}](/tpl/images/0223/5248/f04d5.png)