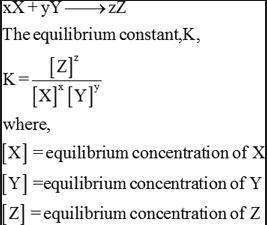
An aqueous solution of acetic acid is found to have the following equilibrium concentrations at 25 c:
[ch3cooh] = 1.65 x 10^-2 m; [h+] = 5.44 x 10^-4 m; and [ch3coo-] = 5.44 x 10^-4 m. calculate the equilibrium constant kc for the ionization of acetic acid at 25 c. the reaction is
ch3cooh -> h+ + ch3coo-

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3


Chemistry, 23.06.2019 00:10, Rubendelarosa1529
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
An aqueous solution of acetic acid is found to have the following equilibrium concentrations at 25 c...
Questions in other subjects:

Mathematics, 28.05.2020 16:58


English, 28.05.2020 16:58



Physics, 28.05.2020 16:59

Health, 28.05.2020 16:59

Business, 28.05.2020 16:59

Mathematics, 28.05.2020 16:59

Mathematics, 28.05.2020 16:59

![Kc=\frac{[H+] [CH3COO-]}{[CH3COOH]}](/tpl/images/0223/5183/bf641.png)