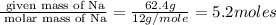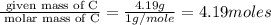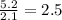Chemistry, 05.09.2019 17:10 samsmith666
Measurements show that unknown compound x has the following composition:
element mass %
carbon 62.4
hydrogen 4.19
oxygen 33.2
write the empirical chemical formula of x?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 22.06.2019 22:00, notearslefttocry14
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 23.06.2019 01:20, hflores0001
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
Measurements show that unknown compound x has the following composition:
element mass %
...
element mass %
...
Questions in other subjects:

Mathematics, 24.12.2019 17:31

Health, 24.12.2019 17:31


Geography, 24.12.2019 17:31


English, 24.12.2019 17:31



Chemistry, 24.12.2019 17:31

Chemistry, 24.12.2019 17:31

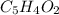 .
.