
Chemistry, 05.09.2019 16:20 gamerhunter425
The four lines observed in the visible emission spectrum of hydrogen tell us that: select one: a. the hydrogen molecules they came from have the formula h4. b. we could observe more lines if we had a stronger prism. c. there are four electrons in an excited hydrogen atom. d. only certain energies are allowed for the electron in a hydrogen atom. e. the spectrum is continuous.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, monnn91351
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 00:00, queenpaige2015
Which samples do the atoms have the least kinetic energy
Answers: 2
You know the right answer?
The four lines observed in the visible emission spectrum of hydrogen tell us that: select one: a....
Questions in other subjects:

Computers and Technology, 28.06.2019 07:00


Biology, 28.06.2019 07:00

Computers and Technology, 28.06.2019 07:00




History, 28.06.2019 07:00


Physics, 28.06.2019 07:00

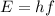 Where h is Planck's constant and f is the frequency of the photon. The frequency of a photon tells us the colour of the light, different colours have different frequencies, and white light contains photons of all frequencies in the visible range. But the light emitted by Hydrogen in a low pressure tube that is excited by an electric current contains only photons from a few frequencies.
Where h is Planck's constant and f is the frequency of the photon. The frequency of a photon tells us the colour of the light, different colours have different frequencies, and white light contains photons of all frequencies in the visible range. But the light emitted by Hydrogen in a low pressure tube that is excited by an electric current contains only photons from a few frequencies. 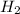 molecules, and even so, the great energy levels in the tube destroy the molecule leaving only single free H atoms.
molecules, and even so, the great energy levels in the tube destroy the molecule leaving only single free H atoms.

