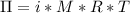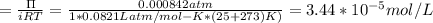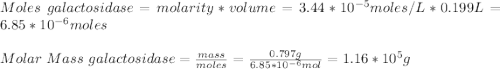Chemistry, 05.09.2019 16:10 elizabethseoane1829
A0.797 g sample of β‑galactosidase is dissolved in water to make 0.199 l of solution, and the osmotic pressure of the solution at 25 ∘c is found to be 0.853 mbar. calculate the molecular mass of β‑galactosidase.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, alexusnicole817
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 13:00, rome58
Lab reagent, hypothesis test. a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl. these six measurements are assumed to be an srs of all possible measurements from solution. they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution. carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1
You know the right answer?
A0.797 g sample of β‑galactosidase is dissolved in water to make 0.199 l of solution, and the osmoti...
Questions in other subjects:

Mathematics, 08.03.2021 21:50

Mathematics, 08.03.2021 21:50






Arts, 08.03.2021 21:50

Mathematics, 08.03.2021 21:50

History, 08.03.2021 21:50