
Chemistry, 05.09.2019 16:10 dorothybean
The combustion of propane (c3h8) produces co2 and h2o: c3h8(g) 5o2(g)→3co2(g) 4h2o(g) the reaction of 2.5 mol of o2 with 4.6 mol of c3h8 will produce mol of h2o.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1

Chemistry, 22.06.2019 05:50, mrylenastewart
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2
You know the right answer?
The combustion of propane (c3h8) produces co2 and h2o: c3h8(g) 5o2(g)→3co2(g) 4h2o(g) the reaction...
Questions in other subjects:



Mathematics, 15.12.2021 22:40

Biology, 15.12.2021 22:40

History, 15.12.2021 22:40



Mathematics, 15.12.2021 22:40

Social Studies, 15.12.2021 22:40

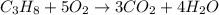
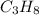 = 4.6
= 4.6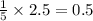 moles of oxygen
moles of oxygen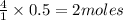 of water
of water


