Using the standard enthalpies of formation, what is the standard enthalpy of reaction?
co(g)...

Chemistry, 05.09.2019 02:30 kaylienguyen
Using the standard enthalpies of formation, what is the standard enthalpy of reaction?
co(g)+h2o(g)⟶co2(g)+h2(g)
the formation values are as follows:
δhf (co(g)) = −110.525kj/mol
δhf (co2(g)) = −393.509kj/mol
δhf (h2o(g)) = −241.818kj/mol
δhf (h2(g)) = 0

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 21:20, skyemichellec
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 11.05.2021 20:00





Mathematics, 11.05.2021 20:00

Mathematics, 11.05.2021 20:00

Social Studies, 11.05.2021 20:00


Mathematics, 11.05.2021 20:00

![\sum [n_{i}\times \Delta H_{f}(product)_{i}]-\sum [n_{j}\times \Delta H_{f}(reactant)_{j}]](/tpl/images/0223/2160/eef77.png)
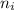 and
and 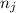 are number of moles of i-th product and j-th reactant in balanced reaction respectively.
are number of moles of i-th product and j-th reactant in balanced reaction respectively.![[(1\times \Delta H_{f}(CO_{2})_{g})]+[(1\times \Delta H_{f}(H_{2})_{g})]-[(1\times \Delta H_{f}(CO)_{g})]-[(1\times \Delta H_{f}(H_{2}O)_{g})]](/tpl/images/0223/2160/f000a.png)
![[(1\times -393.509]+[(1\times 0]-[(1\times -110.525]-[(1\times -241.818]](/tpl/images/0223/2160/cf695.png) kJ = -41.166 kJ
kJ = -41.166 kJ

