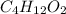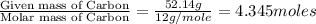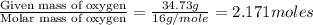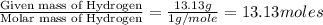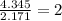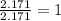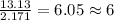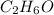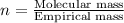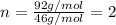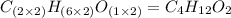Chemistry, 05.09.2019 01:10 fernandaretanaoxwln0
Determine the empirical formula for a compound that contains c, h and o. it contains 52.14% c and 34.73% o by mass. what is the molecular formula if the molar mass is 92 g/mol?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, ian2006huang
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3



Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2
You know the right answer?
Determine the empirical formula for a compound that contains c, h and o. it contains 52.14% c and 34...
Questions in other subjects:

English, 04.11.2020 20:10




Mathematics, 04.11.2020 20:10