
Chemistry, 05.09.2019 00:20 kimloveswim
The combustion of titanium with oxygen produces titanium dioxide: ti(s) + o2(g) → tio2(s) when 2.060 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00 °c to 91.60 °c. in a separate experiment, the heat capacity of the calorimeter is measured to be 9.84 kj/k. the heat of reaction for the combustion of a mole of ti in this calorimeter is kj/mol.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3


Chemistry, 23.06.2019 03:30, tamariarodrigiez
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
The combustion of titanium with oxygen produces titanium dioxide: ti(s) + o2(g) → tio2(s) when 2.06...
Questions in other subjects:




Arts, 07.04.2021 22:50

Mathematics, 07.04.2021 22:50



Biology, 07.04.2021 22:50

Mathematics, 07.04.2021 22:50



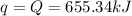
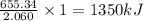 of heat
of heat of heat
of heat


