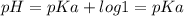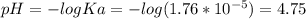Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 23.06.2019 02:50, igraha17
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
The ka of acetic acid is 1.76 × 10-5. the ph of a buffer prepared by combining 50.0 ml of 1.00 m pot...
Questions in other subjects:

Mathematics, 18.02.2021 02:50


English, 18.02.2021 02:50

Mathematics, 18.02.2021 02:50

World Languages, 18.02.2021 02:50

Chemistry, 18.02.2021 02:50

Mathematics, 18.02.2021 02:50

Spanish, 18.02.2021 02:50

English, 18.02.2021 02:50

![pH = pKa + log\frac{[A-]}{[HA]}](/tpl/images/0222/7686/59e2b.png)
![pH = pKa + log\frac{[CH3COOK]}{[CH3COOH]}](/tpl/images/0222/7686/826e4.png)