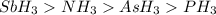Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kiki197701
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3


Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1
You know the right answer?
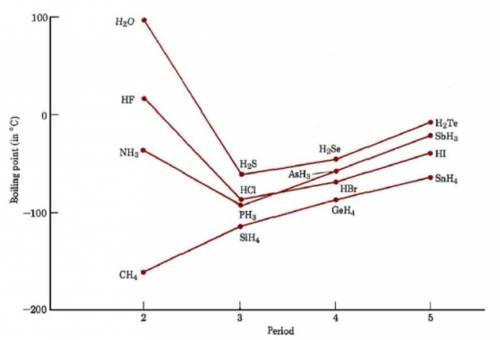
The hydrides of group 5a are nh3, ph3, ash3, and sbh3. arrange them from highest to lowest boiling p...
Questions in other subjects:

Mathematics, 13.02.2022 14:00


Mathematics, 13.02.2022 14:00

Mathematics, 13.02.2022 14:00



Mathematics, 13.02.2022 14:00

Mathematics, 13.02.2022 14:00


Social Studies, 13.02.2022 14:00