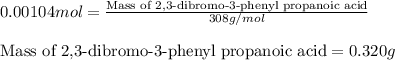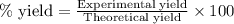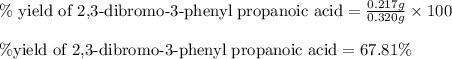Suppose a student started with 155.0 mg of trans-cinnamic acid, 458 mg of pyridinium tribromide, and 2.35 ml of glacial acetic acid. after the reaction and workup, the student ended up with 0.2170 g of brominated product. calculate the student\'s theoretical and percent yields.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 23.06.2019 05:30, Dallas3506
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
Suppose a student started with 155.0 mg of trans-cinnamic acid, 458 mg of pyridinium tribromide, and...
Questions in other subjects:

English, 11.04.2021 08:50


Mathematics, 11.04.2021 08:50

Chemistry, 11.04.2021 08:50



Mathematics, 11.04.2021 08:50

English, 11.04.2021 08:50

Arts, 11.04.2021 08:50

English, 11.04.2021 08:50

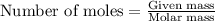 .....(1)
.....(1)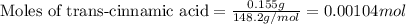
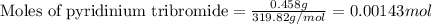
![\text{trans-cinnamic acid}+\text{pyridinium tribromide}\xrightarrow[]{CH_3COOH}\text{2,3-dibromo-3-phenyl propanoic acid}](/tpl/images/0222/4310/95be0.png)
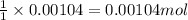 of pyridinium tribromide
of pyridinium tribromide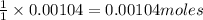 of 2,3-dibromo-3-phenyl propanoic acid
of 2,3-dibromo-3-phenyl propanoic acid