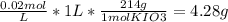Chemistry, 04.09.2019 03:20 JosefineRubino2204
You wish to prepare 1 l of a 0.02 m potassium iodate solution. you require that the final concentration be within 1% of 0.02 m and that the concentration must be known accurately to the fourth decimal place. how would you prepare this solution? specify the glassware you would use, the accuracy needed for the balance, and the ranges of acceptable masses of kio3 that could be used.(a) to make this solution (ideally) you would need grams of potassium iodide dissolved in enough water to make up 1 l of solution. fill in the blank(b)what is the least accurate balance that could be used to make this solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:40, Islandgirl67
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b. zinc7.14,c. copper 8.92,d. lead 11.34
Answers: 2

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 22:10, preachersgirl5
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
You wish to prepare 1 l of a 0.02 m potassium iodate solution. you require that the final concentrat...
Questions in other subjects:


Geography, 01.09.2020 23:01


Physics, 01.09.2020 23:01






Mathematics, 01.09.2020 23:01