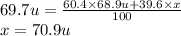Chemistry, 04.09.2019 03:20 hosteenimport21
The element gallium has an atomic mass of 69.7 u and consists of two stable isotopes gallium-69 and gallium-71. the isotope gallium-69 has a mass of 68.9 u and a percent natural abundance of 60.4 %. the isotope gallium-71 has a percent natural abundance of 39.6 %. what is the mass of gallium-71

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2


Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
The element gallium has an atomic mass of 69.7 u and consists of two stable isotopes gallium-69 and...
Questions in other subjects:


Biology, 08.04.2020 01:21








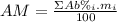
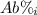 : percent natural abundance of each isotope
: percent natural abundance of each isotope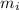 : mass of each isotope
: mass of each isotope