
Chemistry, 04.09.2019 02:30 peytonbrien2002
Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. when 4.51 g of magnesium ribbon burns with 6.92 g of oxygen, a bright, white light and a white, powdery product are formed. enter the balanced chemical equation for this reaction. be sure to include all physical states.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, gonzalesalexiaouv1bg
Using complete sentences, explain how to predict the products and balance the reaction between sulfuric acid and potassium hydroxide.
Answers: 1

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1


You know the right answer?
Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. when 4...
Questions in other subjects:


World Languages, 03.03.2020 04:51





History, 03.03.2020 04:52



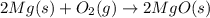
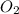 is present in gaseous state, and MgO is present in solid state.
is present in gaseous state, and MgO is present in solid state.


