Chemistry, 04.09.2019 02:30 dzepeda061
2n2h4(g)+ n2o4(g) à3 n2(g) + 4 h2o(g) when 8.0 g of n2h4(32 g mol-1) and 18.4 g of n2o4(92 g mol-1) are mixed together and react according to the equation above, what is the maximum mass of h2o that can be produced?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, lwattsstudent
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

You know the right answer?
2n2h4(g)+ n2o4(g) à3 n2(g) + 4 h2o(g) when 8.0 g of n2h4(32 g mol-1) and 18.4 g of n2o4(92 g mol-1)...
Questions in other subjects:



History, 09.11.2020 17:00







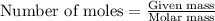 .....(1)
.....(1)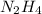 :
: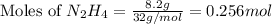
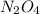 :
: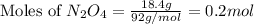
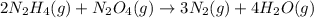
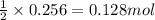 of
of 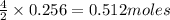 of water
of water