
Chemistry, 04.09.2019 02:20 jeanetteelliotp9ru6m
Consider two gases, a and b, each in a 1.0-l container with both gases at the same temperature and pressure. the mass of gas a in the container is 0.34 g and the mass of gas b in the container is 0.48 g.
a. which gas sample has the most molecules present? b. which gas sample has the largest average kinetic energy? c. which gas sample has the fastest average velocity? d. how can the pressure in the 2 containers be equal to each other since the larger gas b molecules collide with the container walls more forcefully?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
Consider two gases, a and b, each in a 1.0-l container with both gases at the same temperature and p...
Questions in other subjects:

Business, 28.07.2019 10:00



Geography, 28.07.2019 10:00


Biology, 28.07.2019 10:00


Geography, 28.07.2019 10:00


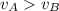 D) compensation between force and velocity.
D) compensation between force and velocity.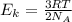 , where R is the ideal gas constant, T is the temperature and
, where R is the ideal gas constant, T is the temperature and 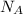 is the Avogadro´s number. All this values are the same in both gases thus they have the same kinetic energy.
is the Avogadro´s number. All this values are the same in both gases thus they have the same kinetic energy.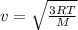 , where M is the molecular mass, if we want to know wich gas has fastest velocity is enough to know the rate between two velocities.
, where M is the molecular mass, if we want to know wich gas has fastest velocity is enough to know the rate between two velocities.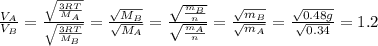 , due to rate is greater than 1
, due to rate is greater than 1 


