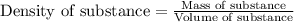Chemistry, 03.09.2019 23:20 DrippyGanja
Follow the steps provided in the simulation to add water to the graduated cylinder, select one of the three samples (copper, silver, or gold), set its mass to the values given in the statements below, find its volume, and calculate its density. to save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density
a) 11.2 g gold and 14.9 g gold
b) 20.2 g silver and 20.2 g copper
c) 15.4 g gold and 18.7 g silver
d) 15.2 g copper and 50.0 g copper

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, brookemcelhaney
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 17:30, katherineweightman
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 23:30, shukriabdisabrie
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
Follow the steps provided in the simulation to add water to the graduated cylinder, select one of th...
Questions in other subjects:

History, 24.06.2019 22:30



Mathematics, 24.06.2019 22:30

Physics, 24.06.2019 22:30



Physics, 24.06.2019 22:30