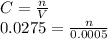Chemistry, 03.09.2019 21:30 champions2k19
A25.0-ml sample containing cu2+ gave an instrument signal of 25.2 units (corrected for a blank). when exactly 0.500 ml of 0.0275 m cu(no3)2 was added to the solution, the signal increased to 45.1 units. calculate the molar concentration of cu2+ assuming that the signal was directly proportional to the analyte concentration. skoog, douglas a.. principles of instrumental analysis (p. 20). brooks cole. kindle edition.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, mimithurmond03
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 04:50, shonnybenskin8
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 06:00, hdjsjfjruejchhehd
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 11:00, hannah5143
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2
You know the right answer?
A25.0-ml sample containing cu2+ gave an instrument signal of 25.2 units (corrected for a blank). whe...
Questions in other subjects:






History, 19.12.2019 23:31

English, 19.12.2019 23:31

Mathematics, 19.12.2019 23:31

History, 19.12.2019 23:31

Physics, 19.12.2019 23:31