Chemistry, 03.09.2019 20:30 montoyaricardo3550
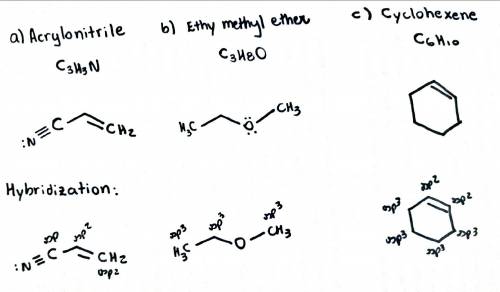
Draw the structures for the following molecules (show all lone pairs): a) acrylonitrile, c3h3n, which contains a carbon-carbon double bond and a carbon-nitrogen triple bond. b) ethyl methyl ether, c3h8o, which contains an oxygen atom bonded to two carbons. c) cyclohexene, c6h10, which contains a ring of six atoms and one carbon-carbon double bond. d) determine the hybridization, the shape, the bond angle and the polarity of each of the structures.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2



Chemistry, 22.06.2019 18:00, LuvieAnn1886
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
Draw the structures for the following molecules (show all lone pairs): a) acrylonitrile, c3h3n, whic...
Questions in other subjects:



Chemistry, 05.08.2021 23:40


History, 05.08.2021 23:40