Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mayamabjishovrvq9
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Asolution contains 0.775 g ca2 0.775 g ca2 in enough water to make a 1925 ml1925 ml solution. what i...
Questions in other subjects:

French, 05.01.2021 09:50




Mathematics, 05.01.2021 09:50

SAT, 05.01.2021 09:50


Mathematics, 05.01.2021 09:50

Mathematics, 05.01.2021 09:50

Mathematics, 05.01.2021 14:00


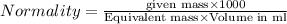
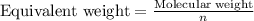
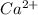 , n =2
, n =2