
Chemistry, 03.09.2019 05:10 kjmccarty02
Determine the direction in which the following reaction is spontaneous at 25oc: mg2+(aq) + k(s) < > mg(s) + k+(aq) determine the equilibrium constant k and go using the cell potential for this reaction and which will be the anode and cathode?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 13:00, rome58
Lab reagent, hypothesis test. a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl. these six measurements are assumed to be an srs of all possible measurements from solution. they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution. carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1
You know the right answer?
Determine the direction in which the following reaction is spontaneous at 25oc: mg2+(aq) + k(s) <...
Questions in other subjects:

Mathematics, 12.09.2019 21:30

English, 12.09.2019 21:30

Spanish, 12.09.2019 21:30

Mathematics, 12.09.2019 21:30


Mathematics, 12.09.2019 21:30


Chemistry, 12.09.2019 21:30

History, 12.09.2019 21:30

Advanced Placement (AP), 12.09.2019 21:30

 and
and  of the reaction is
of the reaction is 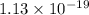 and -108080 J respectively.
and -108080 J respectively. 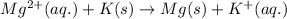

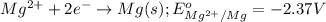
 of the reaction, we use the equation:
of the reaction, we use the equation:

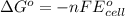


![25^oC=[273+25]=298K](/tpl/images/0221/6855/6a9f9.png)



