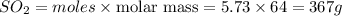Chemistry, 03.09.2019 03:10 lovemusic4
Before the introduction of chlorofluorocarbons, sulfur dioxide (entha; py of vapourization, 6.00 kcal/mol) was used in household refrigrators. what mass of so2 must be evoparated o remove as much heat as evaporation of 1.00 kg of ccl2f2(enthalpy of vapourization is 17.4 kj/mol)?
the vapourition reactions for so2 and ccl2f2 re so2 (i) ? so2 and ccl2f(i) ? ccl2f2(g), respectively

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, marcusajns
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3
You know the right answer?
Before the introduction of chlorofluorocarbons, sulfur dioxide (entha; py of vapourization, 6.00 kca...
Questions in other subjects:

Mathematics, 08.01.2021 17:40


Mathematics, 08.01.2021 17:40

Mathematics, 08.01.2021 17:40


Mathematics, 08.01.2021 17:40

Mathematics, 08.01.2021 17:40


Mathematics, 08.01.2021 17:40

Mathematics, 08.01.2021 17:40

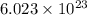 of particles.
of particles.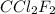 = 17.4 kJ/mol
= 17.4 kJ/mol 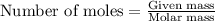
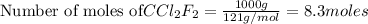
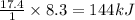
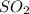 = 6.0 kcal/mol =
= 6.0 kcal/mol = 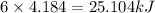 (1kcal=4.184kJ)
(1kcal=4.184kJ)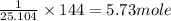 of
of