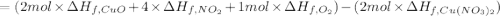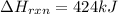The decomposition of copper(ii) nitrate on heating is endothermic reaction. 2cu(no3)2(s) → 2c10(s) + 4no2(g) + o2(g) calculate the enthalpy change for this reaction using the following enthalpy changes of formation. ah! [cu(no3)2) = -302.9 kj mol? ah, (cuo) = -157.3 kj mol? . ah[no2) = +33.2 kj mol.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, juansantos7b
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
The decomposition of copper(ii) nitrate on heating is endothermic reaction. 2cu(no3)2(s) → 2c10(s) +...
Questions in other subjects:










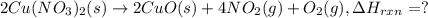
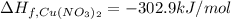
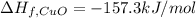
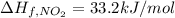
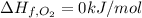 (standard state)
(standard state)![\Delta H_{rxn}=\sum [\Delta H_f(product)]-\sum [\Delta H_f(reactant)]](/tpl/images/0221/5790/84aad.png)
 =
=