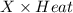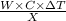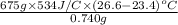When a 0.740-g sample of trinitrotoluene (tnt), c7h5n2o6, is burned in a bomb calorimeter, the temperature increases from 23.4 to 26.9 c the heat capacity of t he calorimeter is 534 j/c, and it contains 675 ml of water. how much heat was produced by the combusttion of the tnt sample?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, elizediax8683
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3


Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
When a 0.740-g sample of trinitrotoluene (tnt), c7h5n2o6, is burned in a bomb calorimeter, the tempe...
Questions in other subjects:



English, 09.03.2021 16:40


English, 09.03.2021 16:40

Physics, 09.03.2021 16:40


Mathematics, 09.03.2021 16:40

History, 09.03.2021 16:40

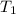 =
= 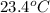
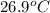
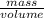
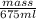
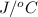
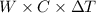 =
=