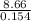In a coffee-cup calorimeter, 140.0 ml of 1.1 m and 140.0 ml of 1.1 m are mixed. both solutions were originally at 25.0°c. after the reaction, the final temperature is 32.4°c. assuming that all the solutions have a density of 1.0 and a specific heat capacity of 4.18 j/°c ⋅ g, calculate the enthalpy change for the neutralization of by . assume that no heat is lost to the surroundings or to the calorimeter. enthalpy change = kj/mol

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 11:30, ansuaprajita1506
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
In a coffee-cup calorimeter, 140.0 ml of 1.1 m and 140.0 ml of 1.1 m are mixed. both solutions were...
Questions in other subjects:

Mathematics, 05.05.2020 00:21

Mathematics, 05.05.2020 00:21

English, 05.05.2020 00:21

Geography, 05.05.2020 00:21


Mathematics, 05.05.2020 00:21