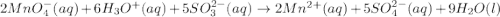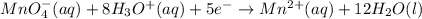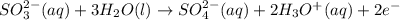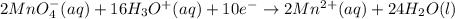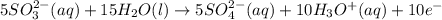Chemistry, 03.09.2019 01:10 littledudefromacross
Balance the following ionic equation for a redox reaction, using whole number coefficients. mno−4(aq)+so2−3(aq)+h3o+(aq)⟶mn2+(a q)+so2−4(aq)+h2o(l)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 05:00, Angelanova69134
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 15:00, makaylajones74pdxtrk
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Balance the following ionic equation for a redox reaction, using whole number coefficients. mno−4(aq...
Questions in other subjects:


Mathematics, 23.07.2021 03:10


Mathematics, 23.07.2021 03:10


Mathematics, 23.07.2021 03:10