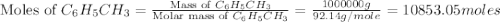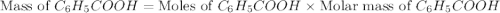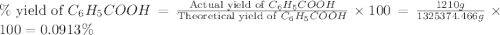Chemistry, 02.09.2019 23:20 aide1234564
Toulene, c6h5ch3 is oxidized by air under carefully controlled conditions to benzoic acid, c6h5co2h, which is used to prepare the food perservative sodium benzoate, c6h5co2na. what is the percent yield os a reaction that converts 1000 kg of toulene to 1.21 kg of benzoic acid
2c6 h5 ch3 + 3o2 ? 2c6 h5 co2 h + 2h2o

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 23:00, lulprettyb
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 22.06.2019 23:00, liv467
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 22.06.2019 23:20, svaskeacevilles5477
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
Toulene, c6h5ch3 is oxidized by air under carefully controlled conditions to benzoic acid, c6h5co2h,...
Questions in other subjects:






English, 01.09.2021 14:00

Mathematics, 01.09.2021 14:00

Mathematics, 01.09.2021 14:00

Advanced Placement (AP), 01.09.2021 14:00

 = 1000 kg = 1000000 g
= 1000 kg = 1000000 g = 122.12 g/mole
= 122.12 g/mole