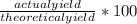Freon-12 ccl2f2, is prepared from ccl4 by reaction with hf. the other product of this reaction is hcl. outline the steps needed to determine the prcent yield of a reaction that produces 12.5 g of ccl2f2 from 32.9 g of ccl4. feron-12 has been banned and is no longer used as a refrigerant because it catalyzes the decomposition of ozone an d has a very long lifetime in the atmosphere. determine the percent yield.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:50, timothymoles
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1


Chemistry, 23.06.2019 04:31, diamondscott9297
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
Freon-12 ccl2f2, is prepared from ccl4 by reaction with hf. the other product of this reaction is hc...
Questions in other subjects:


Social Studies, 23.09.2019 23:40

Biology, 23.09.2019 23:40





Mathematics, 23.09.2019 23:40


Mathematics, 23.09.2019 23:40