

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, ksalinas7404
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 23.06.2019 00:00, juliannasl
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 03:30, uniqueray33
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
Magnesium will burn in air to form both mg3n2 and mgo. what mass of each product would be found if b...
Questions in other subjects:

Mathematics, 30.10.2020 22:30

History, 30.10.2020 22:30




English, 30.10.2020 22:30

Arts, 30.10.2020 22:30

Social Studies, 30.10.2020 22:30

Mathematics, 30.10.2020 22:30

Computers and Technology, 30.10.2020 22:30

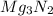 formed is 1.587 grams.
formed is 1.587 grams.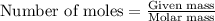 .....(1)
.....(1)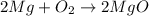
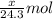
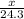 moles of magnesium will produce
moles of magnesium will produce 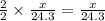 moles of MgO
moles of MgO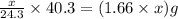
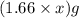 = X grams
= X grams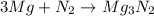
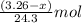
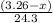 moles of magnesium will produce
moles of magnesium will produce 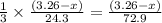 moles of
moles of ![Mg_3N_2=\frac{(3.26-x)}{72.9}\times 101=[(3.26-x)\times 1.38]g](/tpl/images/0221/4247/e57b9.png)
![[(3.26-x)\times 1.38]g](/tpl/images/0221/4247/79581.png) = Y grams
= Y grams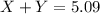
![(1.66\times x)+[(3.26-x)\times 1.38]=5.09\\\\x=2.11g](/tpl/images/0221/4247/08016.png)
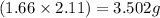
![Mg_3N_2=[(3.26-2.11)\times 1.38]=1.587g](/tpl/images/0221/4247/42984.png)


