
Chemistry, 02.09.2019 21:10 jesusmojica25
Complete and balance the equation for the following acid-base neutralization reaction. if water is used as a solvent, write the reactants and products as aqueous ions. in some cases, there may be more than one correct answer, depending on the amount of reactants used.
(a)mg(oh)2 + hclo4?
(b)so3 + h2o? (assume an excess of water and that the product dissolves)
(c) sro + h2so4?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:20, Mordred9571
Which is true of chemicals? a. things containing chemicals always cost a lot of money. b. chemicals are never dangerous. c. chemicals are in many substances in a home. d. chemicals are rarely found on earth.
Answers: 1

Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 23.06.2019 04:30, Har13526574
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
Complete and balance the equation for the following acid-base neutralization reaction. if water is u...
Questions in other subjects:

English, 06.04.2021 16:40





Mathematics, 06.04.2021 16:40




Spanish, 06.04.2021 16:40


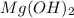 by 2 on reactant side and multiply
by 2 on reactant side and multiply 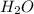 by 2 on product side.
by 2 on product side.




