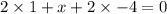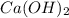Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 18:00, jeepjose58
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
Determine the oxidation states of the elements in the compounds listed. none of the oxygen-containin...
Questions in other subjects:

Mathematics, 20.11.2020 23:10






English, 20.11.2020 23:10

German, 20.11.2020 23:10