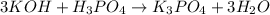Chemistry, 02.09.2019 20:30 makaylarae8781
Classify the following as acid-base reactions or oxidation-reduction reactions:
(a)na2s + hcl -> h2s + 2nacl
(b)2na + 2hcl -> h2 + 2nacl
(c)mg + cl2 = mgcl2
(d)mgo + 2hcl = h2o + mgcl2
(e)k3p+2o2 -> k3po4
(f)3koh +h3po4 -> k3po4 + 3h2o

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 23:30, bxymichelle
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3
You know the right answer?
Classify the following as acid-base reactions or oxidation-reduction reactions:
(a)na2s + hcl...
(a)na2s + hcl...
Questions in other subjects:


Chemistry, 16.09.2021 14:00

Mathematics, 16.09.2021 14:00


History, 16.09.2021 14:00

Chemistry, 16.09.2021 14:00


English, 16.09.2021 14:00

History, 16.09.2021 14:00