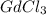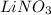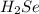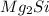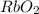Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, laurachealsy923
In an energy pyramid, which level has the most available energy?
Answers: 1


Chemistry, 23.06.2019 03:00, jaidencoolman2866
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1

Chemistry, 23.06.2019 10:30, genyjoannerubiera
4al + 3o2 → 2al2o3 what does the "3" in front of o2 stand for? a) it indicates that there are 5 oxygen atoms after you add the coefficient and the subscript. b) it indicates that that there are are total of 6 oxygen atoms all bonded together as a single molecule. c) it indicates that there are 3 oxygen molecules chemically bonded to each other in the reaction. d) it indicates that there are 3 separate oxygen molecules in the reaction.
Answers: 2
You know the right answer?
Determine the oxidation states of the elements in the following compounds
(a) nai
(b)gdc...
(a) nai
(b)gdc...
Questions in other subjects:








Spanish, 27.09.2019 21:10


Computers and Technology, 27.09.2019 21:10