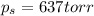Chemistry, 02.09.2019 20:20 genyjoannerubiera
Calculate the vapor pressure lowering of a solution at 100.0 °c that contains 557.1 g of ethylene glycol (molar mass g/mol). the vapor pressure of pure water at t00.0 °c is 760 torr. (2 points) 62.07 g/mol) in 1000.0 g of water (h20 molar mass 18.02 a) 106 torr b) 0.756 torr c) 760 torr (d)186 torr e) none of these

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3


Chemistry, 22.06.2019 09:50, Amandachavez94
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1
You know the right answer?
Calculate the vapor pressure lowering of a solution at 100.0 °c that contains 557.1 g of ethylene gl...
Questions in other subjects:

English, 06.10.2021 17:20

Chemistry, 06.10.2021 17:30



Mathematics, 06.10.2021 17:30



Mathematics, 06.10.2021 17:30

Physics, 06.10.2021 17:30

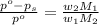
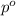 = vapor pressure of pure solvent (water) = 760 torr
= vapor pressure of pure solvent (water) = 760 torr  = vapor pressure of solution = ?
= vapor pressure of solution = ?
 = mass of solute (ethylene glycol) = 557.1 g
= mass of solute (ethylene glycol) = 557.1 g = mass of solvent (water) = 1000.0 g
= mass of solvent (water) = 1000.0 g
 = molar mass of solvent (water) = 18.02 g/mole
= molar mass of solvent (water) = 18.02 g/mole
 = molar mass of solute (ethylene glycol) = 62.07 g/mole
= molar mass of solute (ethylene glycol) = 62.07 g/mole