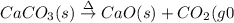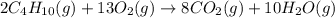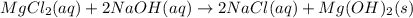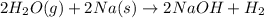Chemistry, 02.09.2019 20:10 codyshs160
Write a balanced molecular equation describing each of the following chemical reactions:
(a)solid calcium carbonate is heated and decomposes to sold calcium oxide and carbon dioxide
(b)gaseous butane, c4h10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor
(c)aaqeous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride
(d)water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, haileywebb8
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

You know the right answer?
Write a balanced molecular equation describing each of the following chemical reactions:
(a)s...
(a)s...
Questions in other subjects:


Biology, 15.07.2019 07:30

Mathematics, 15.07.2019 07:30


Mathematics, 15.07.2019 07:30

Mathematics, 15.07.2019 07:30

Mathematics, 15.07.2019 07:30



Mathematics, 15.07.2019 07:30