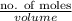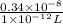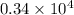Chemistry, 02.09.2019 19:30 elijahjacksonrp6z2o7
The level of mercury in a stream was suspected to be above the minimum considered safe (1part per million by weight). an analysis indicated that the concentration was 0.68 parts per million .assume a density of 1.0 g/ml and calculate the molarity of mercury is the stream.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 23.06.2019 00:30, ejuarez2020
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 07:30, lucas2020197
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2

Chemistry, 23.06.2019 16:30, jules8022
Boron has an average atomic mass of 10.81. one isotope of boron has a mass of 10.012938 and a relative abundance of 19.80 percent . the other isotope has a relative abundance of 80.20 percent what is the mass of that isotope? report two decimal places
Answers: 1
You know the right answer?
The level of mercury in a stream was suspected to be above the minimum considered safe (1part per mi...
Questions in other subjects:

Health, 09.03.2021 14:00

Mathematics, 09.03.2021 14:00

History, 09.03.2021 14:00

Mathematics, 09.03.2021 14:00



Chemistry, 09.03.2021 14:00

History, 09.03.2021 14:00

English, 09.03.2021 14:00

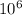 mg of solution.
mg of solution.
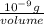
 (as 1 mg = 0.001 g)
(as 1 mg = 0.001 g)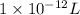 .
.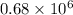 g
g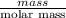
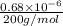
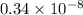 mol
mol