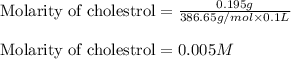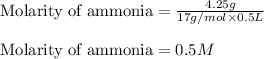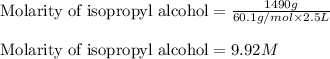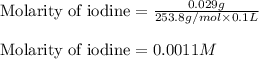Chemistry, 02.09.2019 18:30 allimaycatp8qgaq
Calculate the molarity of each of the following solutions:
(a) 0.195 g of cholestrol, c27h46o, in 0.100 l of serum, the average concentration of cholestrol in human serum
(b) 4.25 gram of nh3 in 0.500 l solution, the concentration of nh3 in household ammonia
(c) 1.49 kg of isopropyl alcohol c3h7oh in 2.50 l of solution the concentration of isopropyl alcohol in rubbing alcohol
(d)0.029 gram of i2 in 0.100 l of solution the solubility of i2 in water at 20 c

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, timiaparker
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2

Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Calculate the molarity of each of the following solutions:
(a) 0.195 g of cholestrol, c27h46o...
(a) 0.195 g of cholestrol, c27h46o...
Questions in other subjects:


Mathematics, 24.09.2021 16:20



Mathematics, 24.09.2021 16:20



Mathematics, 24.09.2021 16:20

Mathematics, 24.09.2021 16:20

Mathematics, 24.09.2021 16:20

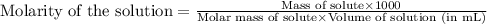 .....(1)
.....(1)