
Chemistry, 02.09.2019 18:20 endreu2005
Calculate the number of moles and the mass of the solute in each of the following solutions: (a) 2.00 l of 18.5 m h2so4, concentrated sulphuric acid
(b)100.0 ml of 3.8 x 10-5 mnacn, the minimum lethal concentration of sodium cyanide in blood serum
(c)5.50 l of 13.3 m h2co, the formaldehyde used to "fix" tissue samples.
(d)325 ml of 1.8 x 10-6 m feso4, the minimum concentration of iron sulphate detectable by taste in drinking water.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, vlactawhalm29
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3


Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

You know the right answer?
Calculate the number of moles and the mass of the solute in each of the following solutions: (a) 2.0...
Questions in other subjects:


Arts, 02.01.2021 23:50



Mathematics, 02.01.2021 23:50





English, 02.01.2021 23:50

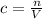
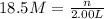
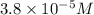 of sodium cyanide
of sodium cyanide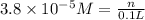
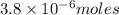 of sodium cyanide
of sodium cyanide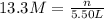
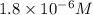 of iron sulphate
of iron sulphate 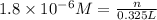
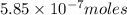 of iron sulfate.
of iron sulfate.

