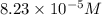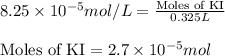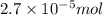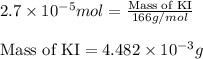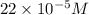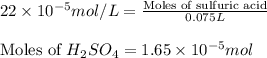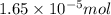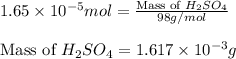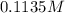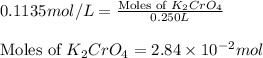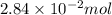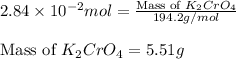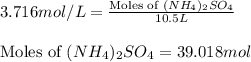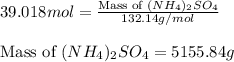Calculate the number of moles and the mass of the solute in each of the following solutions:
(a) 325 ml of 8.23 x 10-5 m kl, a source of iodine in the diet
(b) 75.0 ml of 22 x 10-5 m h2so4, a sample of acid rain
(c) 0.2500 l of 0.1135 m k2cro4 and analytical reagent used in iron assays
(d)10.5 l of 3.716 m (nh4)2so4, a liquid fertilizer

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, laurachealsy923
In an energy pyramid, which level has the most available energy?
Answers: 1


Chemistry, 22.06.2019 03:20, Richwave17
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
Calculate the number of moles and the mass of the solute in each of the following solutions:
...
...
Questions in other subjects:

History, 28.07.2019 10:00








English, 28.07.2019 10:00

Mathematics, 28.07.2019 10:00

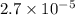 and mass is
and mass is 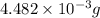
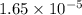 and mass is
and mass is 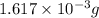
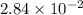 and mass is 5.51 g.
and mass is 5.51 g.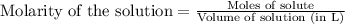 .....(1)
.....(1)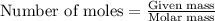 .....(2)
.....(2)