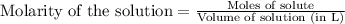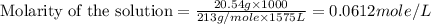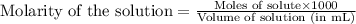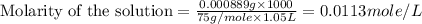Chemistry, 02.09.2019 18:20 aubreyfoster
Determine the molarity for each of the following solution solutions:
(a)1.457 mol of kcl in 1.500 l of solution
(b) 0.515 gram ofh2so4, in 1.00 l of solution
(c) 20.54 g of al(no3)3 in 1575 ml of solution
(d)2.76 kg ofcuso4.5h2o in 1.45 l of solution
(e)0.005653 mol ofbr2 in 10.00 ml of solution
(f) 0.000889 g of glycine, c2h5no2, in 1.05 ml of solution

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, rscvsdfsrysas1857
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
You know the right answer?
Determine the molarity for each of the following solution solutions:
(a)1.457 mol of kcl in...
(a)1.457 mol of kcl in...
Questions in other subjects:

Biology, 10.05.2021 14:00

Mathematics, 10.05.2021 14:00

Geography, 10.05.2021 14:00

English, 10.05.2021 14:00

History, 10.05.2021 14:00


Mathematics, 10.05.2021 14:00


English, 10.05.2021 14:00

English, 10.05.2021 14:00

 solution is, 0.00525 mole/L
solution is, 0.00525 mole/L solution is, 0.0612 mole/L
solution is, 0.0612 mole/L solution is, 7.61 mole/L
solution is, 7.61 mole/L solution is, 0.0565 mole/L
solution is, 0.0565 mole/L solution is, 0.0113 mole/L
solution is, 0.0113 mole/L