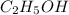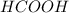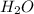Chemistry, 02.09.2019 16:10 kyleejokow
Which contains the greatest mass of oxygen: 0.75 mol of ethanol (c2h5oh), 0.60 mol of formic acid (hco2h) or 1.0 mol
of water (h2o) ? explain why.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 16:50, mathiscool7
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 23.06.2019 01:30, AptAlbatross
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

Chemistry, 23.06.2019 05:00, jjoyner
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
Which contains the greatest mass of oxygen: 0.75 mol of ethanol (c2h5oh), 0.60 mol of formic acid (...
Questions in other subjects:




English, 25.07.2020 04:01


Mathematics, 25.07.2020 04:01

Mathematics, 25.07.2020 04:01

Mathematics, 25.07.2020 04:01