The following data was measured for the reaction above at a constant temperature.
experiment i...

Chemistry, 31.08.2019 05:30 4300224102
The following data was measured for the reaction above at a constant temperature.
experiment initial [a] mol l‐1 initial [b] mol l‐1 initial rate of formation of c (mol l‐1 s‐1)
1 0.10 0.10 1.12 x 10‐3
2 0.10 0.20 2.24 x 10‐3
3 0.20 0.10 4.48 x 10‐3
what is the order of the reaction with respect to a?
what is the order of the reaction with respect to b?
what is the rate law for this reaction?
calculate the rate constant.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:50, deanlmartin
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1



Chemistry, 23.06.2019 04:00, Tiredd7838
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
You know the right answer?
Questions in other subjects:

Chemistry, 27.11.2019 03:31


Mathematics, 27.11.2019 03:31


Mathematics, 27.11.2019 03:31

Biology, 27.11.2019 03:31


Mathematics, 27.11.2019 03:31

Mathematics, 27.11.2019 03:31

Mathematics, 27.11.2019 03:31

![V=k[A]^{2} [B]](/tpl/images/0214/1245/29401.png)
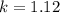
![\frac{[A]_{3}}{[A]_1{}} =2](/tpl/images/0214/1245/57061.png)
![\frac{[V]_{3}}{[V]_1{}} =4](/tpl/images/0214/1245/908c3.png)
![[A]^{2}](/tpl/images/0214/1245/66fd2.png)
![\frac{[B]_{2}}{[B]_1{}} =2](/tpl/images/0214/1245/5cc2d.png)
![\frac{[V]_{2}}{[V]_1{}} =2](/tpl/images/0214/1245/0fa6f.png)
![[B]}](/tpl/images/0214/1245/fe23b.png)
![V=k[A]^{2}[B]](/tpl/images/0214/1245/202fc.png)
![k=\frac{V}{[A]^{2}[B]}](/tpl/images/0214/1245/4243e.png)


