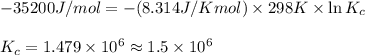What is the equilibrium constant for the reaction:
so2 (g) + no2 (g) â so3 (g) + no (g)
...

Chemistry, 31.08.2019 02:30 cynayapartlow88
What is the equilibrium constant for the reaction:
so2 (g) + no2 (g) â so3 (g) + no (g)
at 298 k? use the following data: r=8.314 j/(k. mol)
substance so2 (g) so3 (g) no2 (g) no (g)
îgo (kj/mol) -300.2 -371 51 86.6
a) 6.8 . 10-7
b) 1.5 . 106
c) 1.014
d) 0.986
e) -35.2

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, drivinghydra
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1



Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 23.06.2019 00:30


History, 23.06.2019 00:30




Computers and Technology, 23.06.2019 00:30


Health, 23.06.2019 00:30

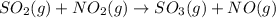
![\Delta G^o_{rxn}=\sum [\Delta G^o_f(product)]-\sum [\Delta G^o_f(reactant)]](/tpl/images/0213/7584/a3f80.png)
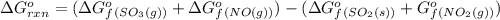
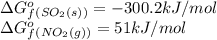
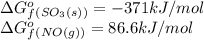
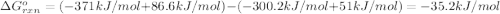
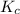 (at 298 K) for given value of Gibbs free energy, we use the relation:
(at 298 K) for given value of Gibbs free energy, we use the relation: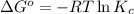
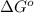 = Gibbs free energy = -35.2 kJ/mol = -35200 J/mol (Conversion factor: 1kJ = 1000J)
= Gibbs free energy = -35.2 kJ/mol = -35200 J/mol (Conversion factor: 1kJ = 1000J)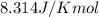
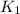 = equilibrium constant at 298 k;
= equilibrium constant at 298 k;