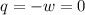Asample consisting of 2 moles he is expanded isothermally at 0 degrees from 5.0dm3 to 20.0dm3. calculate w, q and deltau for each of the following situations: (i) a reversible expansion of the sample. (ii) an irreversible expansion of the sample against a constant external pressure equal to the final pressure of the gas. (iii) a free expansion (against zero external pressure i. e. in a vacuum) of the sample.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1


Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Asample consisting of 2 moles he is expanded isothermally at 0 degrees from 5.0dm3 to 20.0dm3. calcu...
Questions in other subjects:



Geography, 02.04.2021 18:00




Mathematics, 02.04.2021 18:00



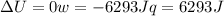
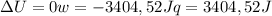
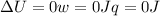
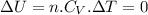 since
since 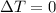
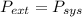 so
so 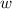 can be calculated by
can be calculated by 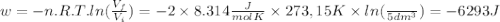
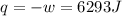
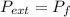
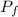 by the law of ideal gases
by the law of ideal gases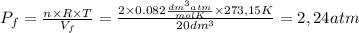
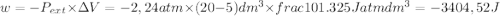
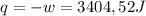
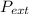 so
so 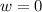 (there's no work at vaccum) and
(there's no work at vaccum) and