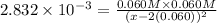Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1


Chemistry, 23.06.2019 07:30, lucas2020197
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2

Chemistry, 23.06.2019 12:30, lindseylewis313
When utilizing a transmission electron microscope, why is it necessary to stain the specimen with heavy metal salts?
Answers: 1
You know the right answer?
Write the equilibrium reactions on a scratch paper, calculate k from ksp and kf and determine the co...
Questions in other subjects:





Mathematics, 18.08.2019 13:20

Mathematics, 18.08.2019 13:20

Mathematics, 18.08.2019 13:20

Mathematics, 18.08.2019 13:20


Chemistry, 18.08.2019 13:20

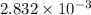 .
.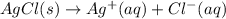
![K_{sp}=1.77\times 10^{-10}=[Ag^+][Cl^-]](/tpl/images/0213/7276/ad131.png) ..(1)
..(1)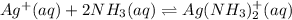
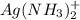 :
:![K_f=1.6\times 10^7=\frac{[Ag(NH_3)_2^{+}]}{[Ag^+][NH_3]^2}](/tpl/images/0213/7276/1553b.png) ..(2)
..(2)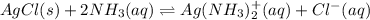
![K=\frac{[Ag(NH_3)_2^{+}][Cl^-]}{[AgCl][NH_3]^2}](/tpl/images/0213/7276/55892.png)
![K=\frac{[Ag(NH_3)_2^{+}][Cl^-]}{[1][NH_3]^2}\times \frac{[Ag^+]}{[Ag^+]}](/tpl/images/0213/7276/23d55.png)
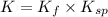 (from 1 and 2)
(from 1 and 2)
![[Ag(NH_3)_2^{+}]](/tpl/images/0213/7276/05dbb.png) = 0.060 M
= 0.060 M![K=\frac{[Ag(NH_3)_2^{+}][Cl^-]}{[1][NH_3]^2}](/tpl/images/0213/7276/bb0d2.png)